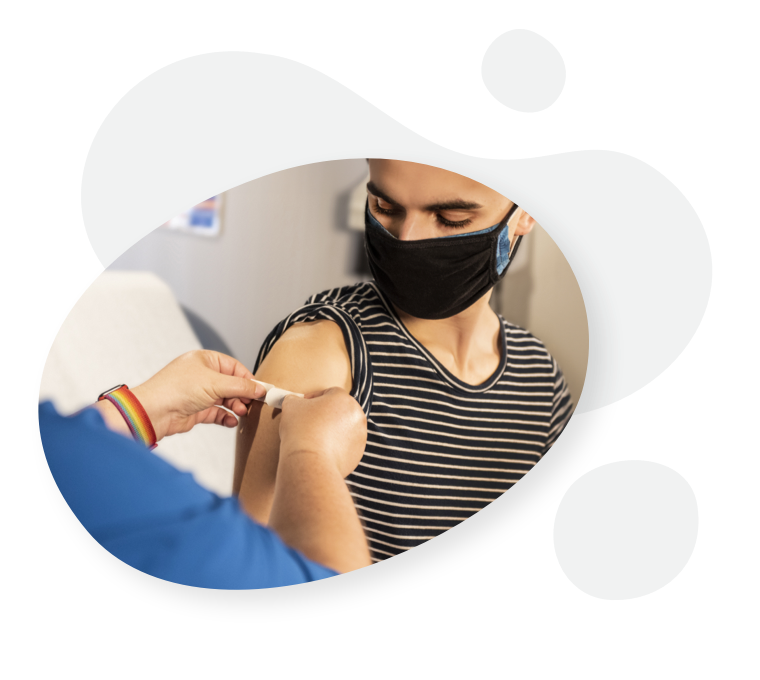
Vaccinations1
RINVOQ (upadacitinib) works by altering your immune responses, so some vaccines should not be given while you are taking RINVOQ. Check with your doctor before you receive any immunisations (such as against herpes zoster). Live vaccines should not be given during RINVOQ treatment, or just before starting RINVOQ treatment.
If you are thinking of travelling overseas or somewhere that will require vaccinations, speak to your doctor. It is recommended that people are brought up to date with all vaccinations before starting RINVOQ therapy.
Pregnancy1,3
RINVOQ should not be taken during pregnancy. If you are pregnant, think you may be pregnant or are planning to have a baby, ask your doctor for advice before taking RINVOQ.
Use effective contraception to avoid becoming pregnant while taking RINVOQ and for at least 4 weeks after your last dose of RINVOQ. Call your doctor straight away if you become pregnant while taking RINVOQ.
You should not take RINVOQ while you are breastfeeding as it is not known if this medicine passes into breast milk.
Travel4
If you are travelling internationally for an extended time, you may want to speak with your doctor or pharmacist about dispensing multiple repeats. This can help ensure you have sufficient medication for your trip. It may not be easy to access RINVOQ in another country.
Also, remember that if you are planning to travel overseas, it is important that you:
Check with the embassy, high commission or consulate of the country you intend to visit that RINVOQ is legal there
Carry a letter from your doctor detailing what RINVOQ is, how much you will be transporting, and stating that it is for your own personal use
Leave RINVOQ in its original packaging so it is clearly labelled with your name and dosage instructions, and to protect it from moisture and heat. Make sure to keep RINVOQ in a cool dry place, below 30°C
You can also download the Travel with RINVOQ letter template and ask your healthcare professional to fill it, so you can have it on hand while travelling.
Refer to the Consumer Medicine Information and speak to your healthcare professional if you have any questions about travelling with RINVOQ.
With the right planning and preparation, it should be possible to travel safely to most destinations. For more information about travelling with RINVOQ, go to Smartraveller website.
If you are concerned about pregnancy or getting vaccinations while taking RINVOQ, speak to your doctor, they can answer your questions.
References: 1. RINVOQ Product Information. 2. Australian Department of Health and Aged Care. Australian Immunisation Handbook: Vaccination for people who are immunocompromised – Secondary (acquired) immunodeficiency due to medical therapies. Available from: immunisationhandbook.health.gov.au/contents/vaccination-for-special-risk-groups/vaccination-for-peoplewho-are-immunocompromised/secondary-acquired-immunodeficiency-due-to-medical-therapies Accessed: August 2025. 3. RINVOQ Consumer Medicine Information. 4. Services Australia. How to manage your PBS medicine overseas. Available from: humanservices.gov.au/individuals/services/medicare/pharmaceutical-benefits-scheme/howmanage-your-pbs-medicine-overseas Accessed: August 2025. AC-005092-00. AU-RNQ-250059. August 2025.